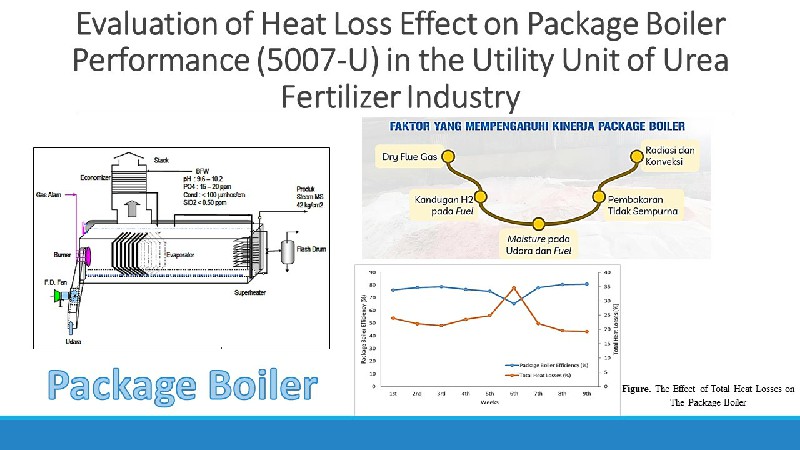
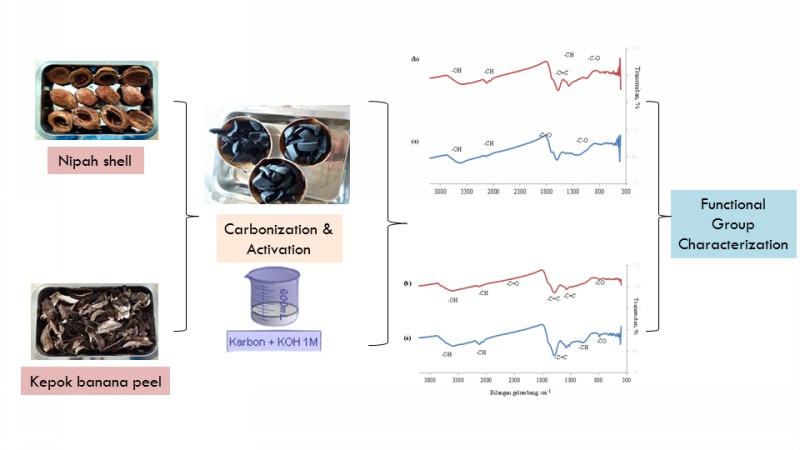
Perbandingan Karbon Aktif-Tempurung Nipah dan Karbon Aktif-Kulit Pisang Kepok Teraktivasi Kalium Hidroksida
Downloads
Penggunaan karbon aktif (activated carbon/AC) semakin luas seperti untuk reaksi kimia, adsorpsi limbah cair dan gas, serta sebagai katalis dalam proses katalitik. AC-tempurung nipah dan AC-kulit pisang kepok telah disintesis dengan aktivator kalium hidroksida (KOH) 0,5 M. Karbonisasi dilakukan dengan furnace pada suhu 300 °C selama 2 jam. Karakterisasi AC dilakukan dengan analisis kadar air, analisis kadar abu, analisis daya serap I2, dan analisis gugus fungsi sebelum proses aktivasi dan setelah proses aktivasi menggunakan FTIR. Nilai kadar air, kadar abu, daya serap terhadap I2berturut-turut adalah 1% ; 9,9%; 1307 mg/g (AC-tempurung nipah) dan 3% ; 7,4% ; 1777 mg/g (AC-kulit pisang kepok), memenuhi kriteria karbon aktif yang telah ditetapkan SNI. Hasil spektra FTIR AC-tempurung nipah dan AC-kulit pisang kepok menunjukkan adanya pergeseran bilangan gelombang serapan gugus -OH setelah aktivasi. Serapan gugus C=C aromatik mengindikasikan telah terbentuknya grafit.
Downloads
Adowei, P., Abia, A., & Spiff, A. (2015). Physicochemical Characteristics of Biocarbons obtained from Nipa Palm (Nypa Fruiticans Wurmb) Leaves. Research Journal of Chemical Sciences, 5(1), 18-26.
Alwarisman, R. (2017). Distribusi, Kerapatan dan Biomassa Nipah (Nypa Fruticans (Thumb.) Wurmb) Di Segara Anakan Cilacap. UNSOED.
Alzaydien, A. S. (2016). Physical, Chemical and Adsorptive Characteristics of Local Oak Sawdust Based Activated Carbons. Asian Journal of Scientific Research, 9(2), 45-56. DOI: 10.3923/ajsr.2016.45.56
Arunakumara, K., Walpola, B. C., & Yoon, M. (2013). Banana Peel: A Green Solution for Metal Removal from Contaminated Waters. Korean J Environ Agric, 32(2), 108-116. DOI: 10.5338/KJEA.2013.32.2.108
Chandana, L., Krushnamurty, K., Suryakala, D., & Subrahmanyam, C. (2019). Low-cost adsorbent derived from the coconut shell for the removal of hexavalent chromium from aqueous medium Materials Today: Proceedings Low-cost adsorbent derived from the coconut shell for the removal of hexavalent chromium from aqueous medium. Materials Today: Proceedings, (August). DOI: 10.1016/j.matpr.2019.04.205
Jubilate, F., Zaharah, T. A., & Syahbanu, I. (2016). Pengaruh Aktivasi Arang dari Limbah Kulit Pisang Kepok Sebagai Adsorben Besi (II) pada Air Tanah. JKK, 5(4), 14-21.
Mongkito, V. H. R., Anas, M., & Bahar, W. P. (2019). Investigating The Effects of Activation Temperature on The Crystal Structure of Activated Charcoal From Palm Bunches ( Arengga Pinnata Merr .). Indonesian Review of Physics, 2(1), 15-21. DOI: 10.12928/irip.v2i1.818
Muniandy, L., Adam, F., Mohamed, A. R., & Ng, E. P. (2014). The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Microporous and Mesoporous Materials, 197, 316-323. DOI: 10.1016/j.micromeso.2014.06.020
Ridhayanti, S. A., & Rusmini. (2020). Pemanfaatan Karbon Aktif dari Limbah Kulit Durian sebagai Adsorben Limbah Industri Tahu di Daerah Sepanjang, Sidoarjo. Jurnal Ilmiah Teknik Kimia, 4(1), 23-31.
Safariyanti, S. J., Rahmalia, W., & Shofiyani, A. (2018). Sintesis dan Karakterisasi Karbon Aktif dari Tempurung Buah Nipah (Nypa fruticans) Menggunakan Aktivator Asam Klorida. Jurnal Kimia Khatulistiwa, 7(2), 41-46.[Sources]
Subiandono, E., Heriyanto, N. M., & Karlina, E. (2011). sebagai Sumber Pangan dari Hutan Mangrove. Buletin Plasma Nutfah, 17(1), 54-60.
Syahrir, I., Samosir, D., Destarini, N. A., & Bariah. (2020). Pemanfaatan Limbah Batang Pisang (Musa paradisiaca L) sebagai Arang Aktif melalui Proses Aktivasi Menggunakan Aktivator NaOH. Prosiding 4th Seminar Nasional Penelitian & Pengabdian Kepada Masyarakat, 54-59. [Sources]
Tamunaidu, P., & Saka, S. (2011). Chemical characterization of various parts of nipa palm (Nypa fruticans). Industrial Crops and Products, 34(3), 1423-1428. DOI: 10.1016/j.indcrop.2011.04.020
Thuan, T. Van, Thi, B., Quynh, P., Duy, T., & Thanh, V. T. (2017). Response surface methodology approach for optimization of Cu2+ , Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surfaces and Interfaces, 6, 209-217. DOI: 10.1016/j.surfin.2016.10.007
Viena, V., Elvitriana, & Nizar, M. (2019). Characterization of Activated Carbon Prepared from Banana Peels: Effect of Chemical Activators on the Adsorption of Gas Emissions. Journal of Physics: Conference Series, 1232(1). DOI: 10.1088/1742-6596/1232/1/012005
Wijaya, D. R. P., Martono, Y., & Riyanto, C. A. (2018). Synthesis and Characterization of Nano Activated Carbon Tea Waste ( Camellia sinensis L .) Viewed from the Content and Ratio of Orthophosphoric Acid. IJCR-Indonesian Journal of Chemical Research, 3(2), 12-21. DOI: 10.20885/ijcr.vol3.iss2.art2
Yuwita, P. E., Mas'udah, K. W., Sunaryono, & Taufiq, A. (2020). Investigation of carbon phase structure of corncob charcoal powder. AIP Conference Proceedings, 2231(April). DOI: 10.1063/5.0002451
Zhang, Y., Zheng, R., Zhao, J., Ma, F., Zhang, Y., & Meng, Q. (2014). Characterization of H3PO4 -Treated Rice Husk Adsorbent and Adsorption of Copper ( II ) from Aqueous Solution. BioMed Research International, 2014(Ii). DOI: 10.1155/2014/496878
Copyright (c) 2022 CHEESA: Chemical Engineering Research Articles

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
With the receipt of the article by CHEESA Editorial Board and the decision to be published, the copyright regarding the article will be transferred to CHEESA Journal.
CHEESA has the right to multiply and distribute the article and every author is not allowed to publish the same article that was published in this journal.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Under the following terms:
Attribution ” You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
NonCommercial ” You may not use the material for commercial purposes.
ShareAlike ” If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original.