Catalyst Lifetime Analysis for High-Temperature Shift Converter (104-D1) at Urea Factory

Downloads
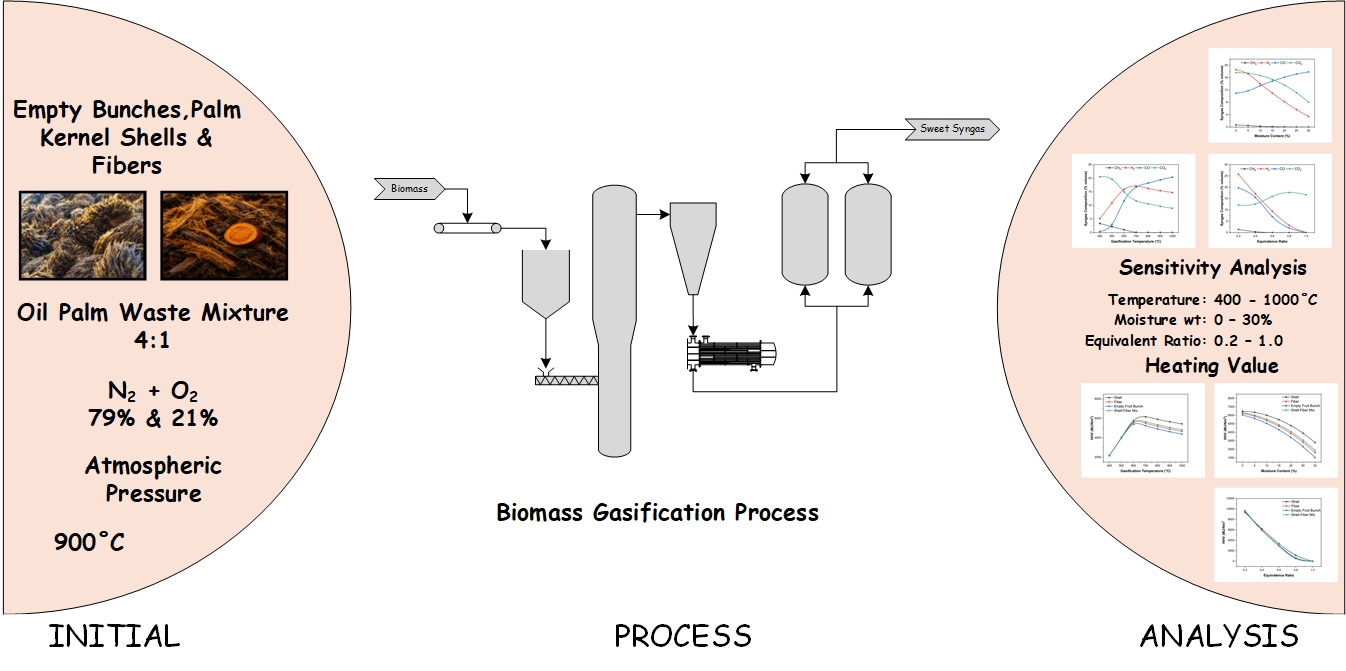
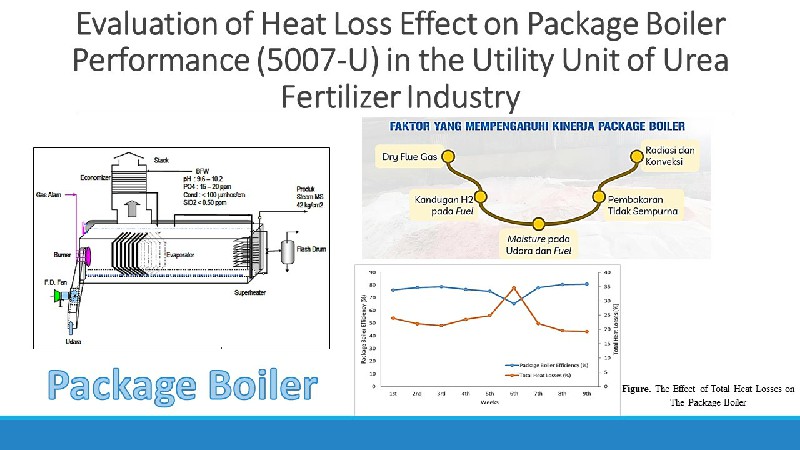
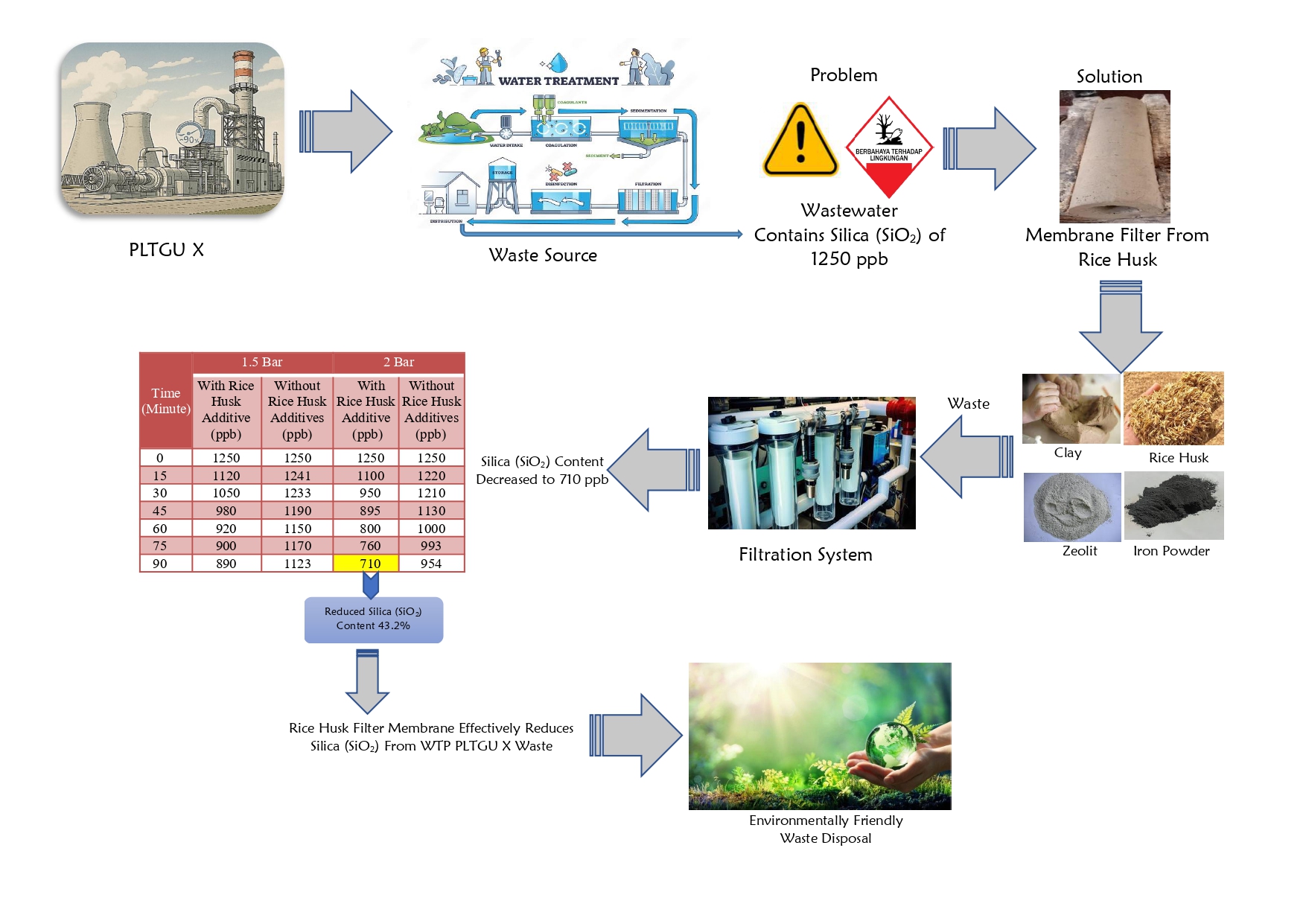
High Temperature Shift (HTS) Converter (104-D1) have a function to convert CO gas into CO2 in the ammonia unit. The presence of CO could be poisoning the catalyst in the ammonia converter. The performance of HTS (104-D1) was ideal if the percentage of CO outlet is above 3.41 mol % dry basis. The performance of HTS (104-D) was influenced by operating conditions (pressure and temperature) and the ratio of steam to carbon. The increase in temperature in HTS (104-D1) can increase in the reaction rate and a decrease in CO conversion due to a decrease in catalyst performance. The research aims to find out how the performance of HTS (104-D1) after Turn Around process (catalyst replacement) that reviewed based on the operating conditions and the resulting CO conversion. The analysis was carried out by comparing the CO conversion from the actual data with the CO conversion from the design data by using the regression equation method to predict the lifetime of the catalyst. CO conversion obtained from the design data is 69.42 %. Conversion of actual data in June, July, August, September and October was 67.43 %; 78.32 %; 77.93 %; 77.75 %; and 77.40 %. The lifetime of the HTS catalyst (104-D1) PUSRI-IIB was estimated to be used up to 5 years after Turn Around process.
Downloads
[1]Aziz, M., TriWijayanta, A., & Nandiyanto, A. B. D. (2020). Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies, 13(12), 3062. DOI: 10.3390/en13123062
[2] PT Pupuk Sriwidjaja Palembang (Pusri). (2023). Anhydrous ammonia. Ammonia Product. Retrieved from https://www.pusri.co.id/en/product/ammonia/anhydrous-ammonia
[3] Felder, R. M., Rousseau, R. W. (2009). Elementary Principles of Chemical Processes. (L. Linda, Ed.)John Wiley & Sons, Inc (4th ed.). United States of America: Laurie Rosatone.
[4] PT Pupuk Sriwidjaja Palembang (Pusri). (2017). Filosofi proses pabrik ammonia PUSRI - IIB kapasitas produksi 2000 MTPD (2nd ed.). Palembang: PT. Pusri Palembang.
[5] Pal, D. B., Chand, R., Upadhyay, S. N., & Mishra, P. K. (2018). Performance of water gas shift reaction catalysts: A review. Renewable and Sustainable Energy Reviews, 93, 549-565. DOI: 10.1016/j.rser.2018.05.003
[6] Kohout, J. (2021). Modified arrhenius equation in materials science, chemistry and biology. Molecules, 26(23), 1-19. DOI: 10.3390/molecules26237162
[7] Ojelade, O. A., & Zaman, S. F. (2021). Ammonia decomposition for hydrogen production: a thermodynamic study. Chemical Papers, 75(1), 57-65. DOI: 10.1007/s11696-020-01278-z
[8] Chen, W. H., & Chen, C. Y. (2020). Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Applied Energy, 258, 1-25. DOI: 10.1016/j.apenergy.2019.114078
[9] Benny, S. (2010). High Temperature Water Gas Shift Catalysts: A Computer Modelling Study. Johnson Matthey Technology Centre. University College London.
[10] Patra, T. K., Mukherjee, S., & Sheth, P. N. (2019). Process simulation of hydrogen rich gas production from producer gas using HTS catalysis. Energy, 173, 1130-1140. DOI: 10.1016/j.energy.2019.02.136
11 Zhang, Z., Wu, Z., Rincon, D., & Christofides, P. D. (2019). Operational safety of an ammonia process network via model predictive control. Chemical Engineering Research and Design, 146, 277-289. DOI: 10.1016/j.cherd.2019.04.004
12 Hughes, R. (1989). Activation, Deactivation and Poisoning of Catalysts. Chemical Engineering Science, 44(8), 1747-1748. DOI: 10.1016/0009-2509(89)80018-1
13 Shekhawat, D., Spivey, J. J., & Berry, D. A. (2011). Fuel Cells: Technologies for Fuel Processing. Fuel Cells: Technologies for Fuel Processing. Amsterdam: Elsevier Science. DOI: 10.1016/C2009-0-20328-X
14 Baboo, P. (2015). Catalyst in Amonia Plant and Their PerformanceEvaluation. National fertilizers Ltd, India.
15 Wahdaniyah, N. (2018). Evaluasi Kinerja Katalis Methanator (106-D) Unit Amonia Operasi Pabrik-2 Pt. Pupuk Kalimantan Timur-Bontang. Politeknik Ati Makassar.
16 Schmidt, L. D. (2005). Engineering of Chemical Reactions. Engineering of Chemical Reactions (2nd ed.). New York: Oxford University Press.
17 Utomo, M. P., & Laksono, E. W. (2007). Tinjauan umum tentang deaktivasi katalis pada reaksi katalisis heterogen. In Prosiding Seminar Nasional Penelitian, Pendidikan, dan Penerapan MIPA (pp. 110-115). Yogyakarta: Universitas Negeri Yogyakarta.
18 Suarsa, I. W. (2017). Teori Tumbukan Pada Laju Reaksi Kimia. Pengembangan Bahan Ajar. Denpasar: Jurusan Teknik Kimia, FMIPA, Universitas Udayana.
19 Dunleavy, J. K. (2006). Sulfur as a catalyst poison. Platinum Metals Review, 50(2), 110. DOI: 10.1595/147106706X111456
20 Uningtya, N. (2018). Evaluasi kinerja katalis pada high temperature shift converter dan low temperature shift converter ( 104-D ) di unit amonia PUSRI-IV. Univesitas Pembangunan Nasional "Veteran" Yogyakarta.
Copyright (c) 2023 CHEESA: Chemical Engineering Research Articles

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
With the receipt of the article by CHEESA Editorial Board and the decision to be published, the copyright regarding the article will be transferred to CHEESA Journal.
CHEESA has the right to multiply and distribute the article and every author is not allowed to publish the same article that was published in this journal.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Under the following terms:
Attribution ” You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
NonCommercial ” You may not use the material for commercial purposes.
ShareAlike ” If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original.