Performance Analysis of Ammonia Converter in Ammonia Unit Factory

Downloads
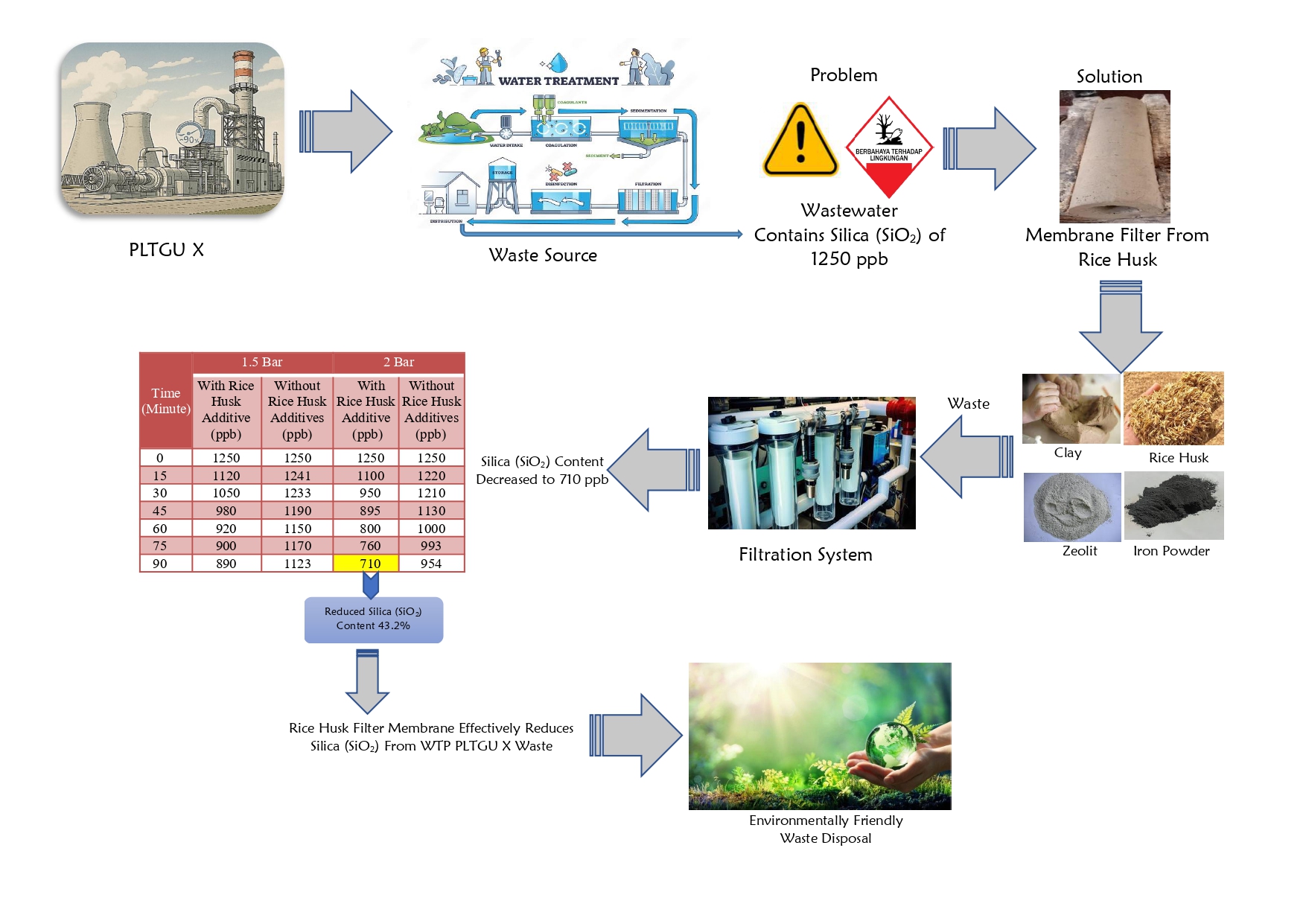
An ammonia converter is a catalyzed reactor that facilitates the synthesis of NH3 (ammonia) from hydrogen (H2) and nitrogen (N2). Several studies have shown that the performance of this reactor significantly influences the operational efficiency and productivity of ammonia plants. Therefore, this study aims to evaluate the performance of an ammonia converter by assessing the effect of operating conditions on the reactant conversion and reaction products using design and actual data. The operating conditions examined included temperature, pressure, ratio of reactants, and inert mole utilized during the NH3 synthesis process. The results showed that the highest NH3 yield of 20.28% was achieved in actual data with 351.5oC temperature, 154.32 kg/cm2 pressure, 3.58 raw material ratio, and 3.57% inert mole (sixth dataset). The performance efficiency of an ammonia converter can be assessed using temperature, reactant ratio, and inert moles, while the pressure factor was insignificant due to dataset fluctuations. Based on the evaluation results, the converter experienced a decrease in performance due to a discrepancy in the existing operating conditions between the design and actual data.
Downloads
Abdurrakhman, S., & Hidayat, M. (2012). Studi Simulasi pada Unit Reformer Primer di PT Pupuk Sriwidjaya Palembang. Jurnal Rekayasa Proses, 6(2), 30.
Aboelkheir, I. M. (2022). An Optimized Chemical and Mechanical Engineering Design of an Ammonia Reactor. Cognizance Journal of Multidisciplinary Studies, 2(1), 10-37. DOI: 10.47760/cognizance.2022.v02i01.002
Agustria, R. M. Y., Al-Azhar., & Putri, R. W. (2019). Evaluasi efisiensi ammonia converter unit ammonia pada industri pupuk urea. Jurnal Teknik Kimia, 25(3), 70-74. DOI: 10.36706/jtk.v25i3.130
Akpa, J. G., & Raphael, N. R. (2014). Optimization of an Ammonia Synthesis Converter. World Journal of Engineering and Technology, 02(04), 305-313. DOI: 10.4236/wjet.2014.24032
Aslan, M. Y., Hargreaves, J. S. J., & Uner, D. (2021). The effect of H2:N2ratio on the NH3synthesis rate and on process economics over the Co3Mo3N catalyst. Faraday Discussions, 229, 475-488. DOI: 10.1039/c9fd00136k
Badescu, V. (2020). Optimal design and operation of ammonia decomposition reactors. International Journal of Energy Research, 44(7), 5360-5384. DOI: 10.1002/er.5286
Cheema, I. I., & Krewer, U. (2020). Optimisation of the autothermal NH3 production process for power-to-ammonia. Processes, 8(1), 1-21. DOI: 10.3390/pr8010038
Demirhan, C. D., Tso, W. W., Powell, J. B., & Pistikopoulos, E. N. (2019). Sustainable ammonia production through process synthesis and global optimization. AIChE Journal, 65(7). DOI: 10.1002/aic.16498
Hakiki, M. N., Hidayat, M., & Sutijan, S. (2017). Simulasi Pengaruh Steam-To-Carbon Ratio Dan Tube Outlet Temperature Terhadap Reaksi Steam Reforming Pada Primary Reformer Di Pabrik Amoniak. Rotor, 10(2), 58. DOI: 10.19184/rotor.v10i2.6393
Klaas, L., Guban, D., Roeb, M., & Sattler, C. (2021). Recent progress towards solar energy integration into low-pressure green ammonia production technologies. International Journal of Hydrogen Energy, 46(49), 25121-25136. DOI: 10.1016/j.ijhydene.2021.05.063
Mao, C., Li, H., Gu, H., Wang, J., Zou, Y., Qi, G., ... Zhang, L. (2019). Beyond the Thermal Equilibrium Limit of Ammonia Synthesis with Dual Temperature Zone Catalyst Powered by Solar Light. Chem, 5(10), 2702-2717. DOI: 10.1016/j.chempr.2019.07.021
Ozturk, M., & Dincer, I. (2021). An integrated system for ammonia production from renewable hydrogen: A case study. International Journal of Hydrogen Energy, 46(8), 5918-5925. DOI: 10.1016/j.ijhydene.2019.12.127
Peng, P., Chen, P., Schiappacasse, C., Zhou, N., Anderson, E., Chen, D., ... Ruan, R. (2018). A review on the non-thermal plasma-assisted ammonia synthesis technologies. Journal of Cleaner Production, 177, 597-609. DOI: 10.1016/j.jclepro.2017.12.229
Qian, J., An, Q., Fortunelli, A., Nielsen, R. J., & Goddard, W. A. (2018). Reaction Mechanism and Kinetics for Ammonia Synthesis on the Fe(111) Surface. Journal of the American Chemical Society, 140(20), 6288-6297. DOI: 10.1021/jacs.7b13409
Rahmatullah, Caesaranty, P. F., & Sari, P. F. (2019). Evaluasi performance ammonia converter Pabrik urea ditinjau dari pengaruh temperatur, tekanan, rasio H2/N2, dan mol inert inlet, serta perhitungan neraca massa dan neraca panas dengan simulator. Jurnal Teknik Kimia, 25(1), 21-30. DOI: 10.36706/jtk.v25i1.17
Siringo-ringo, N. O., Sari, I., & Selpiana. (2019). Evaluasi kinerja ammonia converter pabrik urea ditinjau dari konversi N2 dan H2 dengan menggunakan hysys. Jurnal Teknik Kimia, 25(3), 80-85. DOI: 10.36706/jtk.v25i3.133
Suhan, B. K. M., Hemal, M. N. R, Choudhury, M. A. A. S., & Mazumder, M A. A. (2022). Optimal design of ammonia synthesis reactor for a process industry. Journal of King Saud University - Engineering Sciences, 34(1), 23-30. DOI: 10.1016/j.jksues.2020.08.004
Tripodi, A., Conte, F., & Rossetti, I. (2021). Process Intensification for Ammonia Synthesis in Multibed Reactors with Fe-Wustite and Ru/C Catalysts. Industrial and Engineering Chemistry Research, 60(2), 908-915. DOI: 10.1021/acs.iecr.0c05350
Uline, M. J., & Corti, D. S. (2006). The ammonia synthesis reaction: An exception to the Le Châtelier principle and effects of nonideality. Journal of Chemical Education, 83(1), 138-144. DOI: 10.1021/ed083p138
Wibowo, B. H., & Abdillah, H. L. (2014). Penentuan Tetapan Kecepatan Dan Suhu Reaksi Untuk Memilih Proses Pembuatan Butadien. Majalah Sains dan Teknologi Dirgantara, 9(1), 35-42. Retrieved from LINK
Yancy-Caballero, D., Biegler, L. T., & Guirardello, R. (2015). Optimization of an ammonia synthesis reactor using simultaneous approach. Chemical Engineering Transactions, 43, 1297-1302. DOI: 10.3303/CET1543217
Copyright (c) 2023 CHEESA: Chemical Engineering Research Articles

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
With the receipt of the article by CHEESA Editorial Board and the decision to be published, the copyright regarding the article will be transferred to CHEESA Journal.
CHEESA has the right to multiply and distribute the article and every author is not allowed to publish the same article that was published in this journal.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Under the following terms:
Attribution ” You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
NonCommercial ” You may not use the material for commercial purposes.
ShareAlike ” If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original.