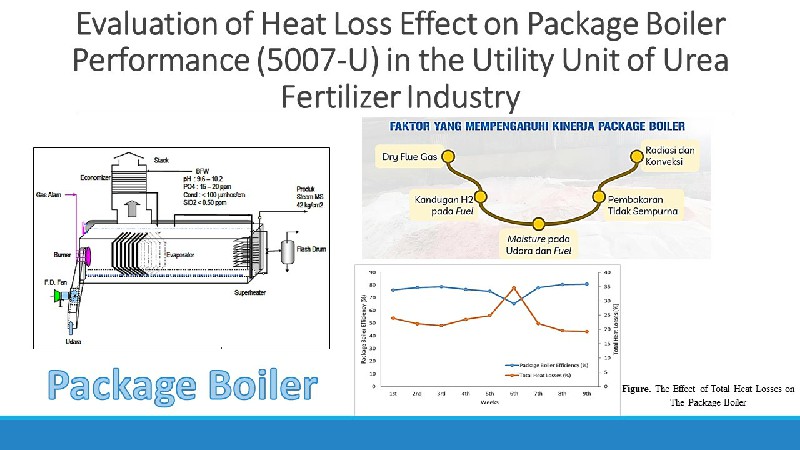
Adsorpsi Methylene Blue pada Nanopartikel Magnetit tersalut Asam Humat: Kajian Isoterm dan Kinetika

Downloads
Nanopartikel magnetit merupakan suatu material dengan sifat magnet yang stabil dan memiliki luas permukaan tinggi. Penyalutan nanopartikel magnetit dengan asam humat (AH) dilaporkan dapat meningkatkan stabilitas, kapasitas adsorpsi, dan kemudahan pemisahan pasca adsorpsi. Penelitian ini bertujuan untuk melakukan sintesis Nanopartikel Magnetit tersalut Asam Humat (NpMAH) dengan metode ko-presipitasi dan menentukan parameter adsorpsinya sebagai adsorben Methylene Blue (MB) dengan metode batch. Keberhasilan sintesis ditunjukkan oleh karakterisasi NpMAH dengan FT-IR, XRD, SEM, dan VSM. Parameter isoterm adsorpsi mengindikasikan bahwa adsorpsi terjadi secara lapis tunggal dengan kapasitas adsorpsi sebesar 56,96 mg/g dan energi adsorpsi sebesar 26,31 kJ/mol pada pH optimum 6,0. Parameter kinetika menunjukkan bahwa adsorpsi mengikuti model kinetika Ho (pseudo orde kedua) dengan konstanta laju adsorpsi (kHo) sebesar 12688,71 g/molmenitdan perhitungan MB yang teradsorpsi pada kesetimbangan (qe) sebesar 2,96—10-5 mol/g. Perhitungan energi adsorpsi menggunakan model kinetika Santosa dan RBS berturut-turut 25,67 kJ/mol dan 41,25 kJ/mol.
Downloads
Adeleke, J. T., Theivasanthi, T., Thiruppathi, M., Swaminathan, M., Akomolafe, T., & Alabi, A. B. (2018). Photocatalytic degradation of methylene blue by ZnO/NiFe2O4 nanoparticles. Applied surface science, 455, 195-200. DOI: 10.1016/j.apsusc.2018.05.184
Barot, N. S., & Bagla, H. K. (2009). Extraction of humic acid from biological matrix - dry cow dung powder. Green Chemistry Letters and Reviews, 2(4), 217-221. DOI: 10.1080/17518250903334290
Basuki, R., Ngatijo, Santosa, S. J., & Rusdiarso, B. (2018). Comparison the new kinetics equation of noncompetitive sorption Cd(II) and Zn(II) onto green sorbent horse dung humic acid (HD-HA). Bulletin of Chemical Reaction Engineering & Catalysis, 13(3), 475-488. DOI: 10.9767/bcrec.13.3.1774.475-488
Basuki, R., Santosa, S. J., & Rusdiarso, B. (2017). Ekstraksi adsorben ramah lingkungan dari matriks biologi : asam humat tinja kuda. Chempublish Journal, 2(1), 13-25.
Basuki, R., Santosa, S. J., & Rusdiarso, B. (2017). The novel kinetics expression of Cadmium (II) removal using green adsorbent horse dung humic acid (Hd-Ha). In AIP Conference Proceedings (Vol. 1823). DOI: 10.1063/1.4978074
Basuki, R., Yusnaidar, Y., & Rusdiarso, B. (2018). Different style of Langmuir isotherm model of non-competitive sorption Zn(II) and Cd(II) onto horse dung humic acid (HD-HA). AIP Conference Proceedings, 2026, 020009. DOI: 10.1063/1.5064969
Chen, Q., Yin, D., Zhu, S., & Hu, X. (2012). Adsorption of cadmium(II) on humic acid coated titanium dioxide. Journal of Colloid and Interface Science, 367(1), 241-248. DOI: 10.1016/j.jcis.2011.10.005
Chen, R. P., Zhang, Y. L., Wang, X. Y., Zhu, C. Y., Ma, A. J., & Jiang, W. M. (2015). Removal of methylene blue from aqueous solution using humic-acid coated magnetic nanoparticles. Desalination and Water Treatment, 55(2), 539-548. DOI: 10.1080/19443994.2014.916233
Dubinin, M. M., & Radushkevich, L. V. (1947). The equation of the characteristic curve of the activated charcoal USSR. Proceedings of the Academy of Sciences, Physical Chemistry Section, 55, 331-337.
El-Sayed, G. O. (2011). Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber. Desalination, 272(1-3), 225-232. DOI: 10.1016/j.desal.2011.01.025
Fitriani, D., Dwita Oktiarni, L., & others. (2015). Pemanfaatan Kulit Pisang Sebagai Adsorben Zat Warna Methylene Blue. GRADIEN: Jurnal Ilmiah MIPA, 11(2), 1091-1095.
Freundlich, H. (1906). Über die Adsorption in Lösungen. Zeitschrift für Physikalische Chemie, 57U(1), 385-470. DOI: 10.1515/zpch-1907-5723
Hikmawati, D. I., & Haqiqi, E. R. (2019). Surface Chemical Structure and Morphological Analysis of Fallen Teak Leaves (Tectona grandis) Powder for Methylene Blue Adsorption. CHEESA: Chemical Engineering Research Articles, 2(1), 42-49. DOI: 10.25273/cheesa.v2i1.4334
Ho, Y. S., McKay, G., Wase, D. A. J., & Forster, C. F. (2000). Study of the sorption of divalent metal ions on to peat. Adsorption Science and Technology, 18(7), 639-650. DOI: 10.1260/0263617001493693
Huda, T., & Yulitaningtyas, T. K. (2018). Kajian adsorpsi methylene blue menggunakan selulosa dari alang-alang. IJCA (Indonesian Journal of Chemical Analysis), 1(01), 9-19. DOI: 10.20885/ijca.vol1.iss1.art2
Illés, E., & Tombácz, E. (2003). The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 230(1-3), 99-109. DOI: 10.1016/j.colsurfa.2003.09.017
Koesnarpadi, S., Santosa, S. J., Siswanta, D., & Rusdiarso, B. (2017). Humic Acid Coated Fe3O4 Nanoparticle for Phenol Sorption. Indonesian Journal of Chemistry, 17(2), 274-283. DOI: https://doi.org/10.22146/ijc.22545
Kustomo, K., & Santosa, S. J. (2019). Studi kinetika dan adsorpsi zat warna kation (metilen biru) dan anion (metil orange) pada magnetit terlapis asam humat. Jurnal Jejaring Matematika dan Sains, 1(2), 64-69.
Labiebah, G., Gunawan, G., Djunaidi, M. C., Haris, A., & Widodo, D. S. (2019). Removal of Methylene Blue Using Used Paper Powder. Jurnal Kimia Sains dan Aplikasi, 22(1), 23-28. DOI: 10.14710/jksa.22.1.23-28
Lagergren, S. (1898). Kungliga svenska vetenskapsakademiens. Handlingar, 24(4), 1-39.
Langmuir, I. (1918). The adsorption of gases on plane surface of glass, mica, and platinum. Journal of the American Chemical Society, 40(9), 1361-1403. DOI: 10.1021/ja02242a004
Lestari, N. D., & Agung, T. (2014). Penurunan TSS Dan Warna Limbah Industri Batik Secara Elektro Koagulasi. Envirotek: Jurnal Ilmiah Teknik Lingkungan, 6(1), 37-44.
Liu, J. F., Zhao, Z. S., & Jiang, G. Bin. (2008). Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environmental Science and Technology, 42(18), 6949-6954. DOI: 10.1021/es800924c
Lu, S., Liu, W., Wang, Y., Zhang, Y., Li, P., Jiang, D., ... Li, Y. (2019). An adsorbent based on humic acid and carboxymethyl cellulose for efficient dye removal from aqueous solution. International Journal of Biological Macromolecules, 135, 790-797. DOI: 10.1016/j.ijbiomac.2019.05.095
Luo, W. J., Gao, Q., Wu, X. L., & Zhou, C. G. (2014). Removal of Cationic Dye (Methylene Blue) from Aqueous Solution by Humic Acid-Modified Expanded Perlite: Experiment and Theory. Separation Science and Technology (Philadelphia), 49(15), 2400-2411. DOI: 10.1080/01496395.2014.920395
Meili, L., Lins, P. V. S., Costa, M. T., Almeida, R. L., Abud, A. K. S., Soletti, J. I., ... Erto, A. (2019). Adsorption of methylene blue on agroindustrial wastes: Experimental investigation and phenomenological modelling. Progress in Biophysics and Molecular Biology, 141, 60-71. DOI: 10.1016/j.pbiomolbio.2018.07.011
Mouni, L., Belkhiri, L., Bollinger, J.-C., Bouzaza, A., Assadi, A., Tirri, A., ... Remini, H. (2018). Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Applied Clay Science, 153, 38-45. DOI: 10.1016/j.clay.2017.11.034
Ngatijo, N., Basuki, R., Nuryono, N., & Rusdiarso, B. (2019). Comparison of Au(III) Sorption on Amine-Modified Silica (AMS) and Quaternary Amine-Modified Silica (QAMS): A Thermodynamic and Kinetics Study. Indonesian Journal of Chemistry, 19(2), 337-346. DOI: 10.22146/ijc.33758
Ngatijo, N., Basuki, R., Rusdiarso, B., & Nuryono, N. (2020). Sorption-desorption profile of Au (III) onto silica modified quaternary amines (SMQA) in gold mining effluent. Journal of Environmental Chemical Engineering, 8(3), 103747. DOI: 10.1016/j.jece.2020.103747
Parvin, S., Rahman, M. W., Saha, I., Alam, M. J., & Khan, M. M. R. (2019). Coconut tree bark as a potential low-cost adsorbent for the removal of methylene blue from wastewater. Desalination and Water Treatment, 146, 385-392. DOI: 10.5004/dwt.2019.23598
Rangabhashiyam, S., Lata, S., & Balasubramanian, P. (2018). Biosorption characteristics of methylene blue and malachite green from simulated wastewater onto Carica papaya wood biosorbent. Surfaces and Interfaces, 10, 197-215. DOI: 10.1016/j.surfin.2017.09.011
Rusdiarso, B., & Basuki, R. (2020). Stability Improvement of Humic Acid as Sorbent through Magnetite and Chitin Modification. Jurnal Kimia Sains dan Aplikasi, 23(5), 152-159. DOI: 10.14710/jksa.23.5.152-159
Rusdiarso, B., Basuki, R., & Santosa, S. J. (2016). Evaluation of Lagergren kinetics equation by using novel kinetics expression of sorption of Zn 2+ onto horse dung humic acid (HD-HA). Indonesian Journal of Chemistry, 16(3), 338-346. DOI: 10.22146/ijc.1158
Sanjaya, H. (2017). Degradasi Methylene Blue Menggunakan Katalis Zno-Peg Dengan Metode Fotosonolisis. EKSAKTA: Berkala Ilmiah Bidang MIPA, 18(02), 21-29. DOI: 10.24036/eksakta/vol18-iss02/45
Santosa, S. J. (2014). Sorption kinetics of Cd(II) species on humic acid-based sorbent. Clean - Soil, Air, Water, 42(6), 760-766. DOI: 10.1002/clen.201200684
Santosa, S. J., Kunarti, E. S., Aprilita, N. H., Wulandari, B., & Bawani, D. N. (2019). Sorption mechanism and performance of peat soil humin for Methylene blue and p-Nitrophenol. Indonesian Journal of Chemistry, 19(1), 198-210. DOI: 10.22146/ijc.33635
Santosa, S. J., Siswanta, D., & Sudiono, S. (2014). Dekontaminasi Ion Logam Dengan Biosarben Berbasis Asam Humat, Kitin, dan Kitosan. UGM PRESS.
Santosa, S. J., Siswanta, D., Sudiono, S., & Utarianingrum, R. (2008). Chitin-humic acid hybrid as adsorbent for Cr(III) in effluent of tannery wastewater treatment. Applied Surface Science, 254(23), 7846-7850. DOI: 10.1016/j.apsusc.2008.02.102
Siyal, A. A., Shamsuddin, M. R., Rabat, N. E., Zulfiqar, M., Man, Z., & Low, A. (2019). Fly ash based geopolymer for the adsorption of anionic surfactant from aqueous solution. Journal of Cleaner Production, 229, 232-243. DOI: 10.1016/j.jclepro.2019.04.384
Stevenson, F. J. (1994). Humus chemistry: genesis, composition, reactions. New York: John Wiley & Sons.
Tempkin, M. I., & Pyzhev, V. (1940). Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR, 12(1), 327-356.
Uddin, M. K., & Nasar, A. (2020). Walnut shell powder as a low-cost adsorbent for methylene blue dye: isotherm, kinetics, thermodynamic, desorption and response surface methodology examinations. Scientific Reports, 10(1), 1-13. DOI: 10.1038/s41598-020-64745-3
Weng, C.-H., & Huang, V. (2015). Application of Fe0 aggregate in ultrasound enhanced advanced Fenton process for decolorization of methylene blue. Journal of industrial and Engineering Chemistry, 28, 153-160. DOI: 10.1016/j.jiec.2015.02.010
Widyaningsih, S., Dwiasi, D. W., & Hidayati, D. (2014). Penurunan konsentrasi zat warna dalam limbah batik menggunakan membaran dari Sargassum sp. Molekul, 9(2), 166-174. DOI: 10.20884/1.jm.2014.9.2.164
Zhang, X., Lei, Q., Wang, X., Liang, J., Chen, C., Luo, H., ... Jiang, J. (2019). Removal of Cr(III) Using Humic Acid-Modified Attapulgite. Journal of Environmental Engineering (United States), 145(6), 1-9. DOI: 10.1061/(ASCE)EE.1943-7870.0001541
Zhang, X., Zhang, P., Wu, Z., Zhang, L., Zeng, G., & Zhou, C. (2013). Adsorption of methylene blue onto humic acid-coated Fe3O4 nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 435, 85-90. DOI: 10.1016/j.colsurfa.2012.12.056
Copyright (c) 2021 CHEESA: Chemical Engineering Research Articles

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
With the receipt of the article by CHEESA Editorial Board and the decision to be published, the copyright regarding the article will be transferred to CHEESA Journal.
CHEESA has the right to multiply and distribute the article and every author is not allowed to publish the same article that was published in this journal.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Under the following terms:
Attribution ” You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
NonCommercial ” You may not use the material for commercial purposes.
ShareAlike ” If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original.