Utilization of Activated Carbon/Magnesium(II) Composites in Decreasing Organic Materials
Downloads
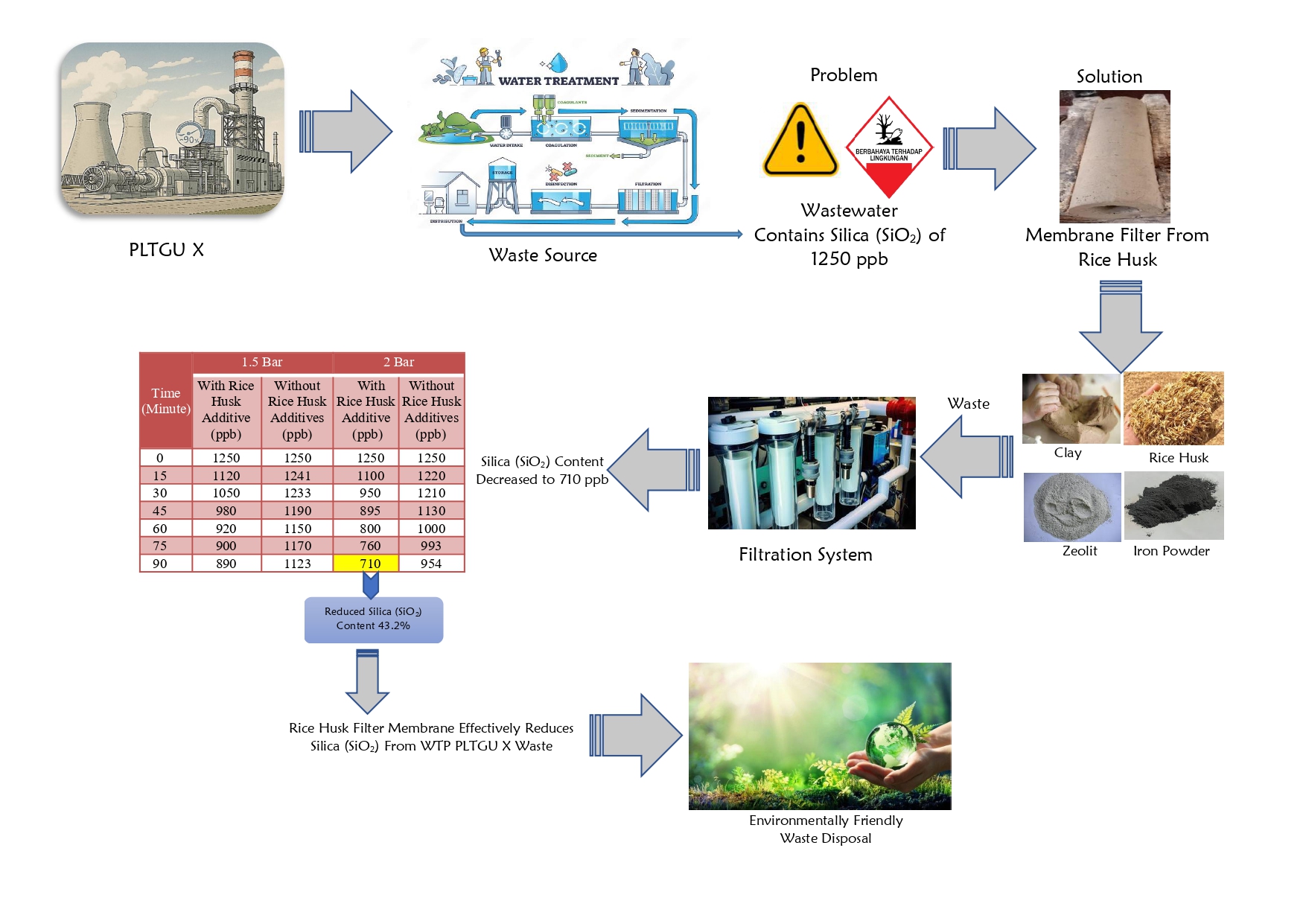
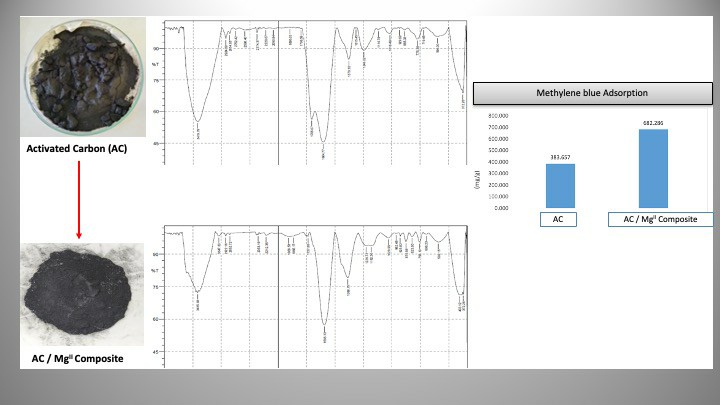
This study aimed to determine the characteristics, adsorption capacity, and isotherm of the adsorbent AC/Mg(II) composite in decreasing organic matter in peat water. Activated carbon was produced from empty fruit bunches of oil palm containing high levels of lignocellulose. Carbon was synthesized through the carbonization process and then activated with CH3COONa. The activated sample was composited with magnesium nitrate hexahydrate through an in-situ method under alkaline conditions using NaOH. The adsorbent AC/Mg(II) composite that had been prepared was characterized using FTIR, showing the presence of Mg-O bonds at the absorption wave number of 403.12 cm-1. The results showed that the moisture content of the adsorbent was lower compared to activated carbon, namely 1.30%. Furthermore, the best mass was 2 g AC/Mg(II) with an adsorption of 2.26 mg/g and an organic matter adsorption percentage of 14.41%. Furthermore, the optimum contact time was 15 minutes with an adsorption of 2.42 mg/g and a percentage of 17.15%. The mechanism occurring in the AC/Mg(II) composite with peat water organic matter followed the Langmuir isotherm equation, which formed a monolayer. The equation gave R2, adsorption capacity (Qo), and adsorption constant (k) values of 0.9994, 0.2340 mg/g, and 0.0047, respectively.
Downloads
Aluyor, E. A. & Badmus, A.M. (2008). COD Removal from Industrial Wastewater using Activated Carbon Prepared from Animal Hons. African Journal of Biotecnology, 7(21), [Sources]
Apriani, R., Faryuni, I, D. & Wahyuni, D. (2013). Pengaruh Konsentrasi Aktivator Kalium Hidroksida (KOH) terhadap Kualitas Karbon Aktif Kulit Durian sebagai Adsorben Logam Fe pada Air Gambut. Jurnal Prisma Fisika, 1 (2), DOI: 10.26418/pf.v1i2.2931
Arh-Hwang, C. & Shin-Meng, C. (2009). Biosorption of Azo Dyes From Aqueous Solution by Glutaraldehyde-Crosslinked Chitosans. Journal of Hazardous Materials, 172, DOI: 10.1016/j.jhazmat.2009.07.104
Arisna, R., Titin, A.Z., & Rudiyansyah. (2016). Adsorpsi Besi dan Bahan Organik pada Air Gambut oleh Karbon Aktif Kulit Durian. JKK, 5(3), 31-39, LINK
Baloga, H., Walanda, D. K. & Hamzah, B. (2019). Pembuatan Arang dari Kulit Nangka (Artocrpus Heterophyllus) Sebagai Adsorben Terhadap Kadmium dan Nikel Terlarut. Jurnal Akademika Kimia, 8(1), 2477-5185, DOI: 10.22487/j24775185.2019.v8.i1.2349
Endaruji, S. & Khaerul, H. (2016). Kajian Adsorpsi remozal yellow FG oleh montmorillonite-kitosan. Integrated Lab Journal, 4(2), [Sources]
Ghalehkhondabi, V., Alireza F. & Keyhan K. (2021). Synthesis and Characterization of Modified Activated Carbon (Magnesium(II)/AC) for Methylene Blue Adsorption:Optimized, Equilibrium Isotherm and Kinetic Studies. IWA Publishing : Water Science and Technology, 83(7), DOI: 10.2166/wst.2021.016
Gova, A.M. & Oktasari, A. (2019). Arang Aktif Tandan Kosong Kelapa Sawit sebagai Adsorben Logam Berat Merkuri (Hg). Prosiding Seminar Nasional Sains dan Teknologi Terapan, Palembang: Universitas Islam Negeri Raden Fatah.
Hustiany, R. & Rahmi, A. (2019). Kemasan Aktif Berbasiskan Arang Aktif Tandan Kosong dan Cangkang Kelapa Sawit. Purwokerto: CV. IRDH.
Istiana, S., Jumaeri & Agung T.P. (2020). Preparasi Arang Aktif Trembesi Magnetit untuk Adsorpsi Senyawa Tannin dalam Limbah Cair. Indo. J. Chem, 9:4, [Sources]
Khaleel, W.A., Sinan, A.S., I.A.M. Alani., M.H.M. & Ahmed. (2019). Magnesium oxide (MgO) thin film as saturable absorber for passively mode locked erbium-doped fiber laser. Elsevier: Optic &Laser Technology, 115, 331-336. DOI: 10.1016/joptlastec.2019.02.042
Langenati, R. M., Mordiono, R., Mustika, D., Wasito, B. & Ridwan. (2012). Pengaruh Jenis Adsorben dan Konsentrasi Uranium Terhadap Pemungutan Uranium Dari Larutan Uranil Nitrat. Jurnal Teknik Bahan Nuklir, 8(2), [Sources]
Maesara, S.A. & Tresna, D.K. (2011). Penyisihan Besi dan Zat Organik Menggunakan Karbon Aktif dari Kulit Durian sebagai Media Filtrasi. Jurnal Teknik Lingkungan, 17(2), DOI: 10.5614/jtl.2012.8.2.7
Mawaddah, D., Zaharah T.A. & Gusrizal. (2014). Penurunan Bahan Organik Air Gambut menggunakaan Biji Asam Jawa. Jurnal Kimia Katulistiwa, 3(1), [Sources]
Muthia, E., Amalia, E.P., Aulia, R., Erdina, L.A.R., Mahmud, M.C.A., Raissa, R., Dede, H.Y.Y. & Muhammad, R.B. (2021). Combination of Coagulation, Adsorption, and Ultrafiltration, Processes for Organic Matter Removal From Peat Water. Sustainability, 14,1, DOI: 10.3390/su14010370
Nga, N.K., Nguyen, T.T.C. & Pham, H.V. (2020). Preparation and Characterization of a Chitosan/MgO Composite for the effective removal of reaction blue 19 dye form aqueous solution. Journal of science: Advanced Materials and Devices, 5(1). DOI: 1016/j.jsamd.2020.01.009
Oladipo, A.A., Olatunji, J.A., Adewale, S.O. & Abimbola, O.A. (2017). Bio-derived MgO Nanopowders for BOD and COD Reduction from Tannery Wastewater. Journal Water Process Engineering, 16, DOI: 10.1016/j.jwpe.2017.01.003
Rahmayani, I., Zaharah, A. T. & Alimuddin, H. A. (2020). Karakterisasi Adsorben Komposit Selulosa-Limbah Karet Alam untuk Penurunan Kadar COD dan Minyak Lemak LCPKS. Jurnal Kimia Khatulistiwa, 8(3), 2303-1077, [Sources]
Rasuli, L., Amir, H.M. (2016). Removal of Humic Acid from Aqueous Solution Using Magnesium(II) Nanoparticles. University of Medical Science, 30(1), DOI: 10.3103/S1063455X16010045
Sopiah, N., Prasetyo, D. & Aviantara, B.D. (2017). Pengaruh Aktivasi Karbon Aktif dari Tandan Kosong Kelapa Sawit Terhadap Adsorpsi Cadmium Terlarut. J. Riset Teknologi Pencegahan Pencemaran Industri, 8(2), DOI: 10.21771/jrtppi.2017.v8.no2.p55-66
Taer, E., Mustika, W. S. & Sugianto. (2016). Pemanfaatan Potensi Tandan Kosong Kelapa Sawit Sebagai Karbon Aktif untuk Pembersih Air Limbah Aktivitas Penambangan Emas. Jurnal Komunikasi Fisika Indonesia, 13(13), DOI: 10.31258/jkfi.13.13.852-858
Copyright (c) 2023 CHEESA: Chemical Engineering Research Articles

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
With the receipt of the article by CHEESA Editorial Board and the decision to be published, the copyright regarding the article will be transferred to CHEESA Journal.
CHEESA has the right to multiply and distribute the article and every author is not allowed to publish the same article that was published in this journal.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Under the following terms:
Attribution ” You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
NonCommercial ” You may not use the material for commercial purposes.
ShareAlike ” If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original.