Pengaruh Variasi Dopan Asam Terhadap Kinerja Baterai Sekunder Polianilina|Zn
Abstract
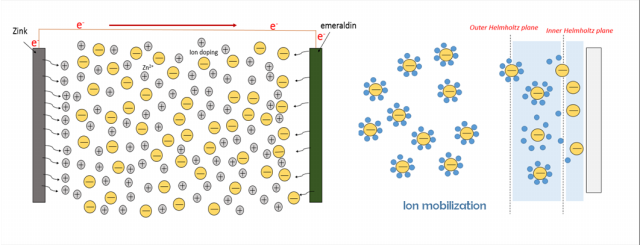
Studi tentang baterai sekunder polianilina(PAni)|Zn telah banyak dikembangkan, tetapi sejauh ini masih belum ada penjelasan mengenai pengaruh asam terhadap kinerja baterai tersebut. PAni disitensis menggunakan metoda elektrodeposisi dengan tegangan 0,7 V selama 30 menit. PAni hasil sintesis dikarakterisasi menggunakan Voltammetri dan Spektroskopi Inframerah. Baterai PAni|Zn didesain dalam bentuk sandwich, kemudian pengukuran kinerja digunakan impedansi serta pengisian-pengosongan. Baterai dengan elektroda PAni-Cl dan PAni-Br mempunyai kinerja yang lebih baik dengan specific capacity saat ke-60 yaitu 55,4 dan 37,4 mAh g-1. Pengukuran impedansi pada baterai dengan elektroda PAni-Cl, PAni-Br, dan PAni HClO4, menunjukan resistance solution (Rs) secara berurutan yaitu 1,38; 2,56; dan 3,03 W dan resistance charge transfer (Rct) 2,24; 2,97; dan 7,71 W. Oleh sebab itu, baterai PAni|Zn dengan dopan HCl menunjukkan kinerja terbaik dibanding dengan asam yang lain.
Keywords
Full Text:
DOWNLOAD PDFReferences
Chang, X., Hu, R., Sun, S., Liu, J., Lei, Y., Liu, T., Dong, L., & Yin, Y. (2018). Sunlight-charged electrochromic battery based on hybrid film of tungsten oxide and polyaniline. Applied Surface Science, 441, 105–112. https://doi.org/10.1016/j.apsusc.2018.02.003
Chen, Y., & Manzhos, S. (2016). Voltage and capacity control of polyaniline based organic cathodes: An ab initio study. Journal of Power Sources, 336, 126–131. https://doi.org/10.1016/j.jpowsour.2016.10.066
Deyab, M. A., & Mele, G. (2019). Polyaniline/Zn-phthalocyanines nanocomposite for protecting zinc electrode in Zn-air battery. Journal of Power Sources, 443, 227264. https://doi.org/10.1016/j.jpowsour.2019.227264
Gao, H., Lu, Q., Yao, Y., Wang, X., & Wang, F. (2017). Significantly Raising the Cell Performance of Lithium Sulfur Battery via the Multifunctional Polyaniline Binder. Electrochimica Acta, 232, 414–421. https://doi.org/10.1016/j.electacta.2017.02.160
Hatchett, D. W., Josowicz, M., & Janata, J. (1999). Acid doping of poly aniline: Spectroscopic and electrochemical studies. Journal of Physical Chemistry B, 103(50), 10992–10998. https://doi.org/10.1021/jp991110z
Huang, J., Tu, J., Lv, Y., Liu, Y., Huang, H., Li, L., & Yao, J. (2020). Achieving mesoporous MnO2@polyaniline nanohybrids via a gas/liquid interfacial reaction between aniline and KMnO4 aqueous solution towards Zn-MnO2 battery. Synthetic Metals, 266, 116438. https://doi.org/10.1016/j.synthmet.2020.116438
Kawashima, H., Okatani, R., Mayama, H., Nakamura, Y., & Fujii, S. (2018). Synthesis of hydrophobic polyanilines as a light-responsive liquid marble stabilizer. Polymer, 148, 217–227. https://doi.org/10.1016/j.polymer.2018.06.039
Kurada, K. V., & De, S. (2018). Polyaniline doped ultrafiltration membranes: Mechanism of membrane formation and pH response characteristics. Polymer, 153, 201–213. https://doi.org/10.1016/j.polymer.2018.08.032
Li, X., Lv, R., Zou, S., Na, B., Liu, P., Ma, Y., & Liu, H. (2019). Polyaniline nanopillars on surface cracked carbon fibers as an ultrahigh-performance cathode for a flexible rechargeable aqueous Zn-ion battery. Composites Science and Technology, 180, 71–77. https://doi.org/10.1016/j.compscitech.2019.05.016
Luo, J., Zhong, W., Zou, Y., Xiong, C., & Yang, W. (2016). Preparation of morphology-controllable polyaniline and polyaniline/graphene hydrogels for high performance binder-free supercapacitor electrodes. Journal of Power Sources, 319, 73–81. https://doi.org/10.1016/j.jpowsour.2016.04.004
Šeděnková, I., Trchová, M., Dybal, J., & Stejskal, J. (2016). Interaction of polyaniline film with dibutyl phosphonate versus phosphite: Enhanced thermal stability. Polymer Degradation and Stability, 134, 357–365. https://doi.org/10.1016/j.polymdegradstab.2016.11.005
Sovizi, M. R., & Fahimi, Z. (2018). Honeycomb polyaniline-dodecyl benzene sulfonic acid (hPANI-DBSA)/sulfur as a new cathode for high performance Li–S batteries. Journal of the Taiwan Institute of Chemical Engineers, 86, 270–280. https://doi.org/10.1016/j.jtice.2018.03.004
Taheri, N. N., Ramezanzadeh, B., Mahdavian, M., & Bahlakeh, G. (2018). In-situ synthesis of Zn doped polyaniline on graphene oxide for inhibition of mild steel corrosion in 3.5 wt.% chloride solution. Journal of Industrial and Engineering Chemistry, 63, 322–339. https://doi.org/10.1016/j.jiec.2018.02.033
Zhang, X., Lin, Q., Zhang, X., & Peng, K. (2018). A novel 3D conductive network-based polyaniline/graphitic mesoporous carbon composite electrode with excellent electrochemical performance. Journal of Power Sources, 401, 278–286. https://doi.org/10.1016/j.jpowsour.2018.08.091
Zhao, C., Jin, Y., Du, X., & Du, W. (2018). In situ prepared amorphous FeCoO-Polyaniline/multiwalled carbon nanotube nanohybrids as efficient oxygen evolution catalysts for rechargeable Zn-air batteries. Journal of Power Sources, 399, 337–342. https://doi.org/10.1016/j.jpowsour.2018.07.111
Zhao, Z., Yu, T., Miao, Y., & Zhao, X. (2018). Chloride ion-doped polyaniline/carbon nanotube nanocomposite materials as new cathodes for chloride ion battery. Electrochimica Acta, 270, 30–36. https://doi.org/10.1016/j.electacta.2018.03.077
Article Metrics
Abstract has been read : 1423 timesDOWNLOAD PDF file viewed/downloaded: 0 times
DOI: http://doi.org/10.25273/cheesa.v3i1.6702
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.



